Docs concerned for the use of Favipiravir for COVID in India
By MYBRANDBOOK
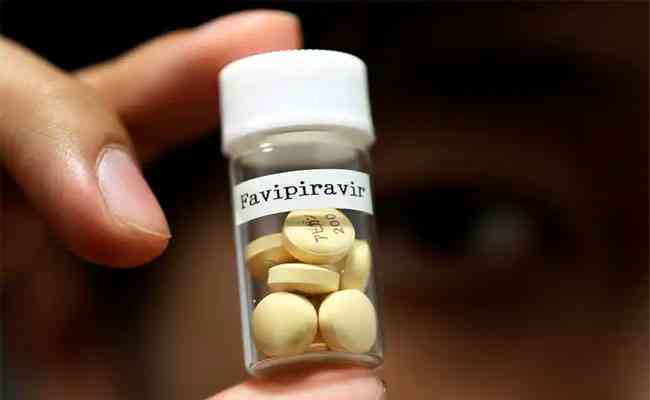
Favipiravir, an antiviral drug for the treatment of mild-to-moderate COVID-19 cases, was launched recently by Glenmark Pharmaceuticals. According to reports, the drug has been launched after it received approval from the Drug Controller General of India to manufacture and market the medicine.
The antiviral drug Favipiravir has been approved in Japan since 2014 for the treatment of novel or re-emerging influenza virus infections. It works by inhibiting viral replication and reducing the viral load in a patient. It is an experimental medicine being repurposed for COVID-19.
According to Glenn Saldanha, Chairman and Managing Director of Glenmark Pharmaceuticals the approval has come at a time when cases in India are spiralling, putting tremendous pressure on our healthcare system.
The drug FabiFlu will be available as a prescription-based medication for Rs 103 per tablet, with the recommended dose being 1800 mg twice daily on day 1, followed by 800 mg twice daily up to day 14.
The drug is not recommended in patients with severe renal, hepatic impairment, and in pregnant and lactating women.
Glenmark has claimed that Favipiravir shows clinical improvements of up to 88% in COVID-19 disease, with a rapid reduction in viral load by 4 days.
Favipiravir is being studied in at least 18 trials around the world as a potential treatment for COVID-19. Earlier last month, Glenmark also announced that it is conducting another clinical trial to evaluate the efficacy of two antivirals Favipiravir and Umifenovir as a combination therapy in moderate hospitalized adult COVID-19 patients in India.
But according to doctors there is insufficient evidence in favour of the medicine. A large portion of doctors don’t feel there is any real requirement of the medicine for mild attacks. They feel there is so much panic and fear around the disease that they will be forced to arrange for the medicine even if they have no funds - when in reality, a mild COVID case may recover either without medication, or with medicines costing no more than Rs 10.


Legal Battle Over IT Act Intensifies Amid Musk’s India Plans
The outcome of the legal dispute between X Corp and the Indian government c...

Wipro inks 10-year deal with Phoenix Group's ReAssure UK worth
The agreement, executed through Wipro and its 100% subsidiary,...

Centre announces that DPDP Rules nearing Finalisation by April
The government seeks to refine the rules for robust data protection, ensuri...
Home Ministry cracks down on PoS agents in digital arrest scam
Digital arrest scams are a growing cybercrime where victims are coerced or ...


ICONS OF INDIA : SRIDHAR VEMBU
Sridhar Vembu is the chief executive officer (CEO) of Zoho Corporation...

Icons Of India : Arjun Malhotra
Arjun Malhotra, the Chairman of Magic Software Inc., is widely recogni...

ICONS OF INDIA : RAJIV MEMANI
As Chair of the EY Global Emerging Markets Committee, Rajiv connects e...


BEL - Bharat Electronics Limited
BEL is an Indian Government-owned aerospace and defence electronics co...

GeM - Government e Marketplace
GeM is to facilitate the procurement of goods and services by various ...

NSE - National Stock Exchange
NSE is the leading stock exchange in India....


Indian Tech Talent Excelling The Tech World - ANJALI SUD, CEO – Tubi
Anjali Sud, the former CEO of Vimeo, now leads Tubi, Fox Corporation�...

Indian Tech Talent Excelling The Tech World - PADMASREE WARRIOR, Founder, President & CEO - Fable
Padmasree Warrior, the Founder, President, and CEO of Fable, is revolu...

Indian Tech Talent Excelling The Tech World - JAY CHAUDHRY, CEO – Zscaler
Jay Chaudhry, an Indian-American technology entrepreneur, is the CEO a...
 of images belongs to the respective copyright holders
of images belongs to the respective copyright holders